veriseq nipt v2
At Illumina our goal is to apply innovative technologies to the analysis of genetic variation and function making studies possible that were not even imaginable just a few years ago. Comprehensive IVD in-lab aneuploidy screening solution providing reagents instruments and software for accurate NIPT results in 26 hours.

Veriseq Nipt Microlab Star Automated Liquid Handling Hamilton Company
Sequencing with the VeriSeq NIPT Solution v2 enables comprehensive insights reducing the need for invasive tests.

. Welcome to Immense Discovery Power. Assign 20 provides visualization of the sequencing reads to assess read diversity. NovaSeq 6000 Sequencing System is by far our most powerful instrument designed to adapt to your needs.
Illumina has launched the VeriSeq NIPT Solution v2 a CE-IVD next-generation sequencing-based approach to noninvasive prenatal testing. The assay provides information about fetal chromosomal status as early as 10. PDF 1 MB Aug 13 2021.
The TG VeriSeq NIPT Sample Prep Kit is used with the VeriSeq NIPT Solution RUO. Assign 20 is also designed to seek read diversity and uses a broad range of reads reducing the likelihood of PCR artifacts contributing to the. VeriSeq NIPT Solution Package Insert Translated into Czech.
VeriSeq NIPT Solution v2 Package Insert 1000000078751 v06 PDF 1 MB Aug 16 2021. Instructions for processing samples with the VeriSeq NIPT Solution v2. It is available as an Illumina Advantage TG product.
P1 reagents are now available for NextSeq 1000NextSeq 2000 Systems offering added flexibility to meet your projects needs. The VeriSeq NIPT Microlab STAR allows a single user to prepare and analyze 48 or 96 samples simultaneously with results in approximately one day compared to two days or longer using other methods. The VeriSeq NIPT Solution v2 is an in vitrodiagnostic test intended for use as a screening test for the detection of genome-wide fetal genetic anomalies from maternal peripheral whole blood specimens in pregnant women of at least 10 weeks gestation.
VeriSeq NIPT Solution v2 Package Insert Translated into Brazilian Portuguese. VeriSeq NIPT Solution v2 provides accurate information about fetal chromosomal status as early as 10 weeks of gestation using a single maternal blood draw. The VeriSeq NIPT Solution v2 is an in vitro diagnostic test intended for use as a screening test for the detection of genome-wide fetal genetic anomalies from maternal peripheral whole blood specimens in pregnant women of at least 10 weeks gestation.
The new version expands the range of chromosomal and sub-chromosomal conditions associated with birth defects that laboratories can screen for. VeriSeq NIPT Solution v2 uses whole-genome sequencing to detect partial duplications and. Product includes components of library preparation sequencing and analysis.
The VeriSeq NIPT Solution v2 is an in vitro diagnostic test intended for use as a screening test for the detection of genome-wide fetal genetic anomalies from maternal peripheral whole blood specimens in pregnant women of at least 10 weeks gestation. Welcome to Immense Discovery Power. VeriSeq NIPT Solution v2 Package Insert 200006957 v00 for Canada.
Instructions for processing samples with the VeriSeq NIPT Solution kit. Served as technical writing project lead for the release and launch of VeriSeq NIPT Solution v2 an end-to-end solution for non-invasive prenatal testing. Hands-free throughput and sample integrity are enabled through powerful features such as eight.
VeriSeq NIPT Solution v2 Package Insert 1000000078751 v06 1 MB. NextSeq 10002000 Reagents. VeriSeq NIPT Solution v2 Package Insert Translated into Brazilian Portuguese.
Enhanced Time Savings and Sample Integrity. Chapter1VeriSeqNIPTSolutionv2 Introduction 1 SystemArchitecture 2 Introduction TheVeriSeqNIPTSolutionv2isaninvitrodiagnostictestintendedforsequencing-basedscreeningforthe. Instructions for using the VeriSeq NIPT Solution v2.
VeriSeq NIPT Solution Package Insert Translated into Danish. NovaSeq 6000 Sequencing System is by far our most powerful instrument designed to adapt to your needs. VeriSeq NIPT Solution v2 Package Insert 200006957 v00 for Canada.
This noninvasive test provides an option to screen for aneuploidy in all autosomes chromosomes X Y and partial deletions and duplications greater than 7 Mb across the genome. VeriSeq NIPT Solution Package Insert 1000000001856 v08 1 MB. Although artifacts can occur in the TruSight HLA v2 assay the assay is optimized to amplify them at a very low rate.
The VeriSeq NIPT Solution v2 is an in vitrodiagnostic test intended for use as a screening test for the detection of genome-wide fetal genetic anomalies from maternal peripheral whole blood specimens in pregnant women of at least 10 weeks gestation. Created edited and updated the Package Insert and Software Guide for the product. All Reproductive Health Products.
Instructions for analyzing assay data using the VeriSeq NIPT Solution v2 software. Equipment Height Width Depth Weight VeriSeqOnsiteServerv2 438 cm 173 in 178 cm 7in 635 cm 25 in 259kg 57lbs VeriSeqNIPT MicrolabSTARwithAutoload 903 cm 356 in 199 cm 783 in 1006 cm 396 in 160kg 353lbs VeriSeqOnsiteServerv2PlacementRequirements PositiontheVeriSeqOnsiteServerv2toallowfor. Illumina Advantage large-scale sequencing products feature lot-specific shipments and testing extended shelf life and advanced change notifications for greater laboratory efficiency.

Illumina Introduces Expanded Version Of Veriseq Nipt Solution Offering More Comprehensive Detection Of Rare Chromosomal Conditions Business Wire

Veriseq Nipt Solution V2 Support

Illumina Inc Ilmn Presents At J P Morgan Healthcare Conference Slide Show Nasdaq Ilmn Seeking Alpha

The Veriseq Nipt Solution Youtube

Illumina And Next Generation Genomic Partner To Launch Veriseq Nipt Solution In Thailand
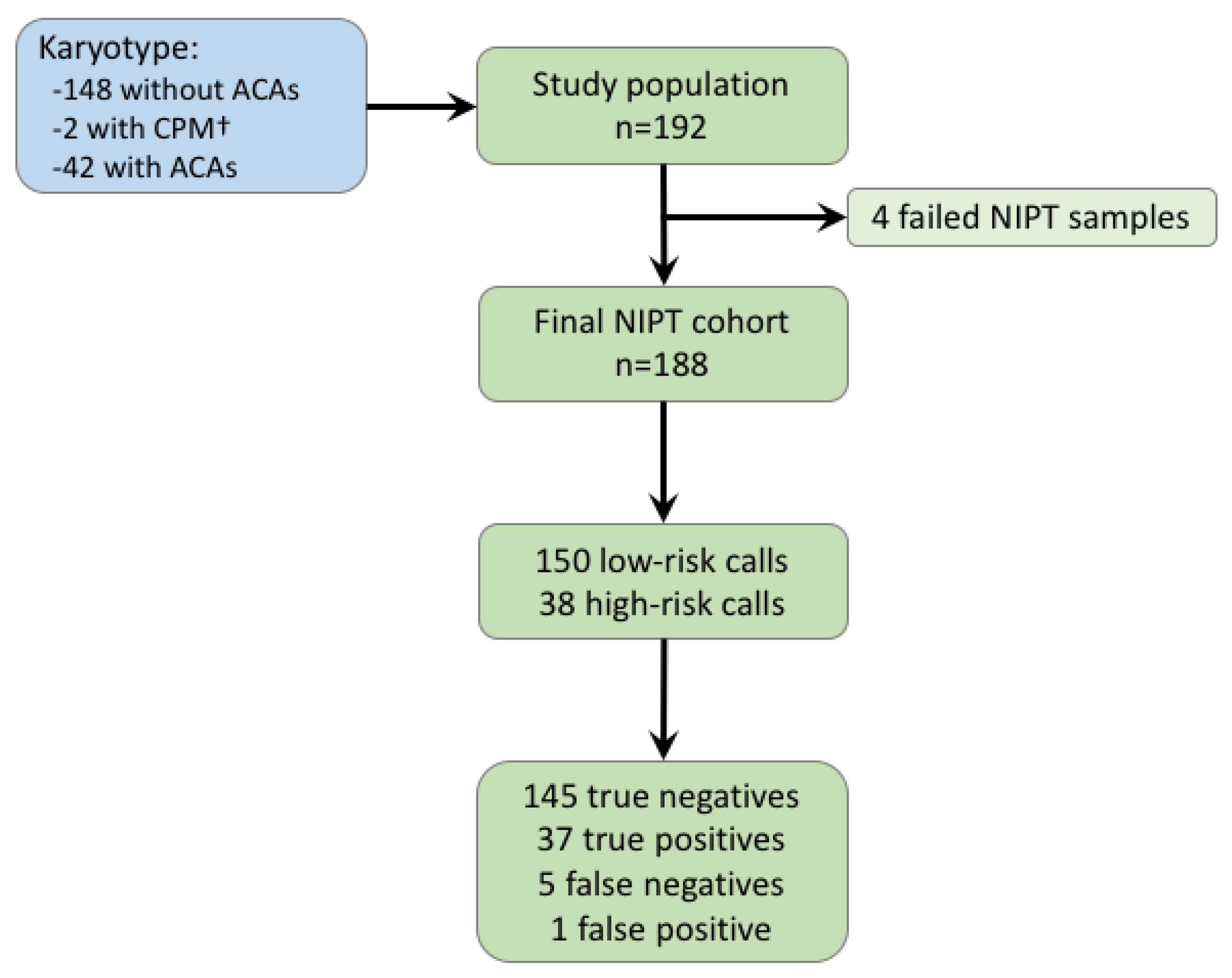
Jcm Free Full Text Strategy For Use Of Genome Wide Non Invasive Prenatal Testing For Rare Autosomal Aneuploidies And Unbalanced Structural Chromosomal Anomalies Html

In Lab Screening With Nipt Turnkey Sample To Results In Your Lab
Veriseq Nipt Solution V2 Comprehensive And Reliable Nipt Solution

Illumina On Twitter Fdesouza Version 2 Of Veriseq Nipt Will Ship In 1h 2019 Adding Karyotype Resolution Across The Genome And Increasing The Number Of Genetic Diseases That Can Be Detected Jpm19
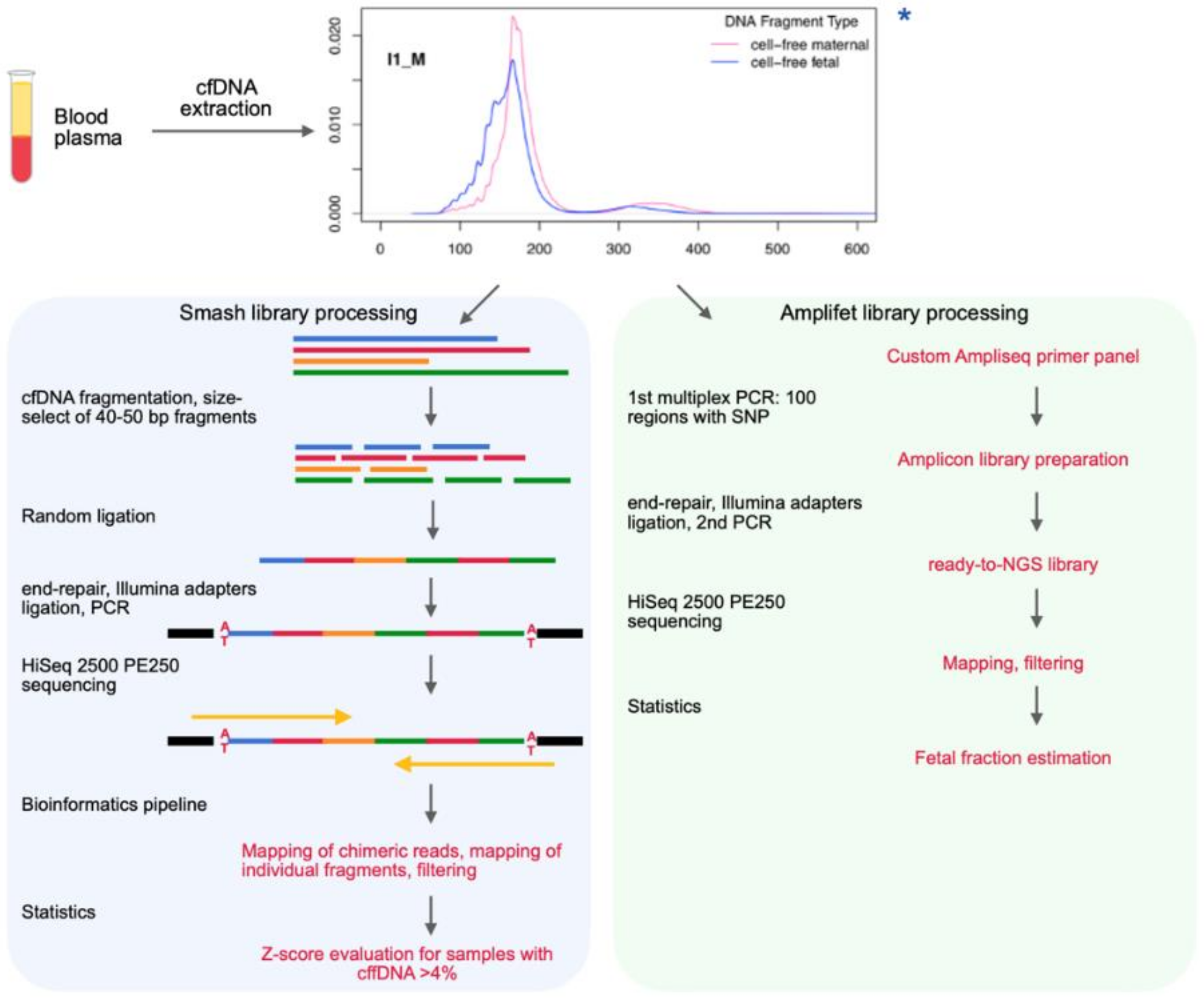
Genes Free Full Text Nipt Technique Based On The Use Of Long Chimeric Dna Reads Html

Illumina S Noninvasive Prenatal Screening Kit Receives Regulatory Approval In S Korea Business Wire

In Lab Screening With Nipt Turnkey Sample To Results In Your Lab
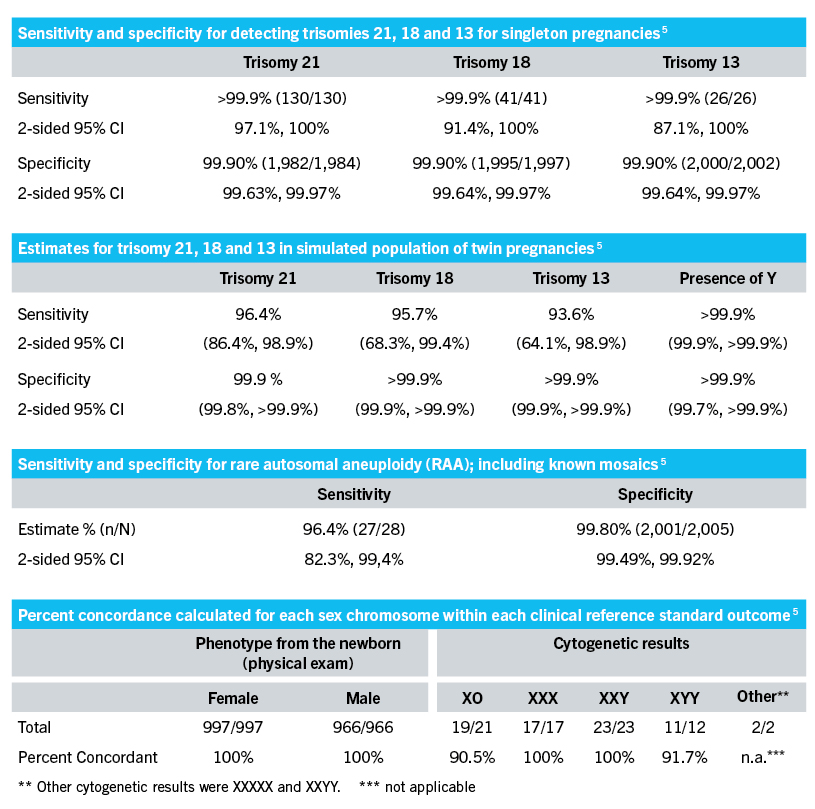
Performance Qualification Praenatest
Veriseq Pgs Kit Miseq Vitrolife
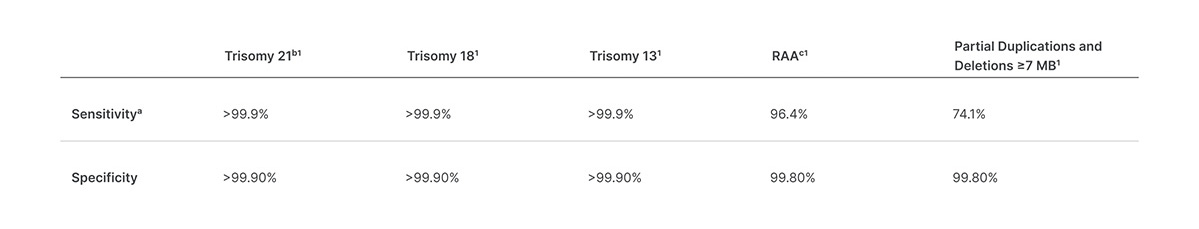
Veriseq Nipt Solution V2 Comprehensive And Reliable Nipt Solution

Veriseq Nipt Solution V2 Comprehensive And Reliable Nipt Solution
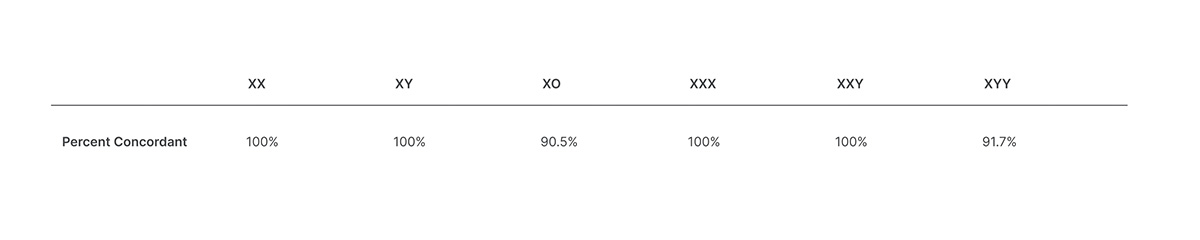
Veriseq Nipt Solution V2 Comprehensive And Reliable Nipt Solution
0 Response to "veriseq nipt v2"
Post a Comment